|

Lab
Diagnostic Testing: Adenovirus
The laboratory
diagnosis of ocular adenovirus infection is a function of the onset
of clinical symptoms. The earlier the conjunctival samples are collected
after clinical onset, the higher likelihood of a positive result.
The adenoviral load of viable virus and antigen decreases over time.
Four tests can be used for laboratory diagnosis: PCR , cell
culture, shell
vial , and EIA (Adenoclone™). In our laboratory we currently only offer PCR for ADV.
Specimen
Collection
PCR
Cell
Culture
Shell
Vial Culture
EIA
(Adenoclone™)
Specimen
Collection
Specimens are directly collected by vigorously swiping the exposed
conjunctiva with a plastic soft-tipped applicator. Cornea samples
are not necessary. Topical anesthetic can be applied to the conjunctiva
but this is optional. Collected samples are placed in 2.0 ml of
viral transport medium. We have had great success with Bartels ChlamTrans™ chlamydial
transport medium and recommend its use. Viral culturettes can also
be used for transportation to the laboratory and these can be transferred
to the viral medium. All laboratory testing can be processed from
the 2.0 ml of chlamydial transport medium. Adenovirus is not a fastidious
virus. It will remain viable under many conditions and collected
samples should be easily transported through mail carriers.
Viral Transport medium
(Click on image to enlarge)
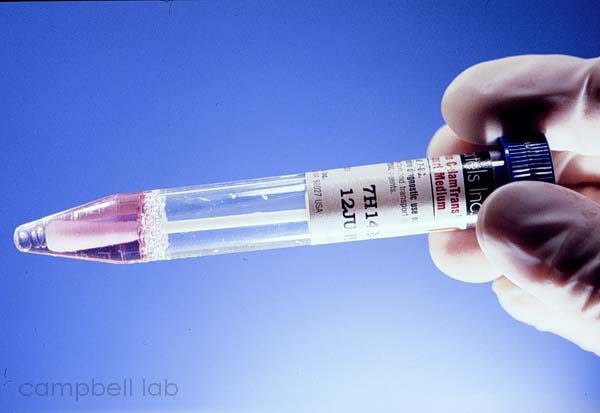 |
PCR
Polymerase Chain Reaction (PCR) is a molecular test that amplifies
specific adenoviral DNA sequences from clinical samples and then
identifies the amplified products with gel techniques. PCR is a
highly sensitive and specific test that can detect adenoviral DNA
from clinical samples. Results can now be obtained within one to
three days. PCR testing can be quite expensive
to the self-pay patient. Make sure the patient has insurance coverage.
Many molecular laboratories offer adenoviral PCR testing.
Cell
culture
The "gold" standard for adenovirus laboratory testing
is cell culture. Collected samples are layered on a monolayer of
cells in test tubes. If present, Adenovirus will present as cytopathic
effect of rounded cells. The cytopathic effect of Adenovirus is
confirmed for the presence of antigen by EIA (Adenoclone™). The best
cell-line for testing Adenovirus is A549. This is a human carcinoma
continuous cell-line. When samples are collected within one to three
days of clinical onset, cell culture generally is positive within
four to seven days. Samples collected after three days may take
one to three weeks to produce cytopathic effect. Cell culture will
confirm an adenovirus diagnosis but it may not provide timely results
for immediate patient care. All virology laboratories can offer
cell culture isolation for Adenovirus.
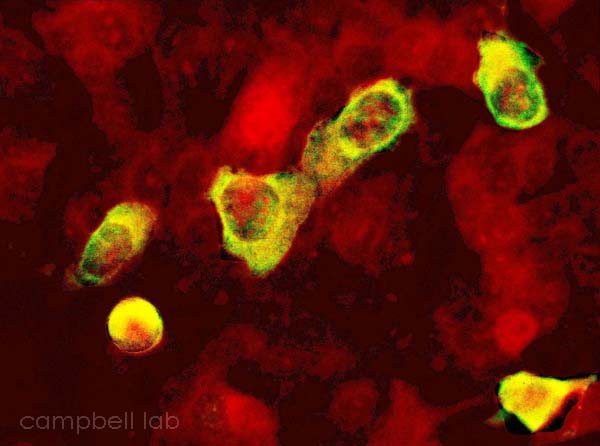
Shell
Vial Culture
Shell vial is another cell culture test but the results are ready
in three days. Vials of A549 cells are inoculated with collected
samples and centrifuged. The vials are then incubated and stained
at day three with immunofluorescent antibodies specific to Adenovirus.
The cells infected with Adenovirus will light up with examination
under a fluorescent microscope. We found that shell vial highly
correlates with standard cell culture especially when samples are
collected within seven days of clinical onset. Shell vial testing
is processed by many virology laboratories, especially those involved
with respiratory virus.
Positive ADV shell vial culture
(Click on image to enlarge)
 |
EIA
(Adenoclone™)
Adenoclone™ is an enzyme immunoassay that can detect adenoviral antigen
from collected ocular specimens. Positive results can be obtained
within 75 minutes. Unfortunately, Adenoclone™ is only 40 to 50 percent
sensitive in detecting adenoviral antigen from clinical specimens.
A high load of antigen is necessary for a positive test and this
correlates with collection within one to three days of clinical
onset. The power of this test is that it does provide rapid results
when tests are positive but negative tests need to be confirmed
with cell culture or shell vial. Adenoclone™ is not widely offered
by diagnostic laboratories.
Positive Adenoclone™
 |

|



