 |

Lab
Diagnostic Testing: Bacteria
The most common detected pathogen from the eye is bacteria. The techniques have
not changed much over the last 40 years although the bacterial nomenclature has changed with genera and species. The areas that are frequently infected with bacteria are the conjunctiva (conjunctivitis), eyelids (blepharitis), cornea (keratitis), and the aqueous and vitreous inside the eye (endophthalmitis). The cornea, aqueous, and vitreous are not inhabited with bacteria. The normal conjunctiva contains no colonizing bacteria but probably is continuously contaminated with flora from the eyelids. The eyelids are colonized with numerous amounts of coagulase negative staphylococci, diphtheroids, and an occasional Staphylococcus aureus, Viridans group Streptococcus, and Gram-negative bacteria.
Media
for Bacterial Isolation
The most important culture media for isolation of bacteria from ocular locations are 1) 5% sheep's blood agar, 2) chocolate agar,
and 3) mannitol salt agar. Most bacteria can be isolated on the 5% sheep's blood, but chocolate agar is necessary to isolate Haemophilus species, and nutritionally variant Streptococcus species that are frequent pathogens of conjunctivitis and keratitis. Neisseria gonorrhoeae also requires chocolate agar. Mannitol salt, if available, is helpful
to the microbiologist for rapidly identifying Staphylococcus isolates. Anaerobic bacteria are rare pathogens of the eye. The most frequent anaerobic pathogen is Cutibacterium acnes that is suspected in chronic endophthalmitis after phaco-cataract extraction surgery. Cutibacterium acnes is most efficiently isolated in enriched thioglycollate, and 5% sheep's blood and chocolate agars incubated anaerobically. When unusual bacterial pathogens (anaerobic bacteria, mycobacteria, nocardia, etc.) are suspected, physicians must make arrangements with the laboratory to assure that the appropriate media is plated and incubated properly.
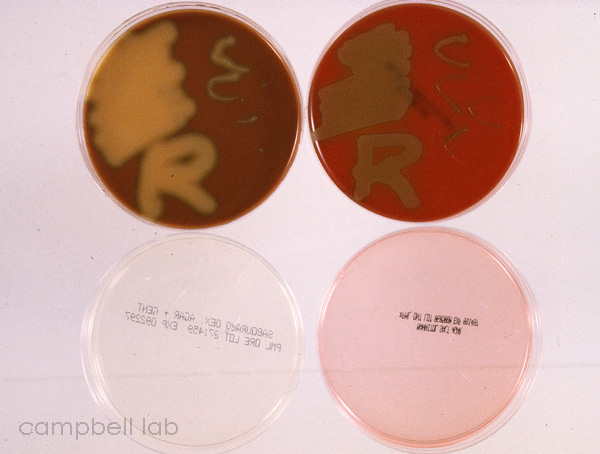
Streptococcus pneumoniae
Choc, blood, sab, mannitol plates
(Click on image to enlarge)
 |
Specimen
Collection: Conjunctivitis and Blepharitis
Ocular specimens from conjunctivitis and blepharitis are easily
collected with soft-tipped applicators (i.e. cotton, Dacron, or
calcium alginate swabs). Prior to collection, the soft-tipped applicators
are moistened with laboratory-supplied broth but any non-preserved
sterile medium (i.e. PBS, BSS) would suffice. Many ophthalmologists
apply a topical anesthetic (0.5% proparacaine) prior to obtaining
a sample but this may not be necessary for most patients presenting
with conjunctivitis and blepharitis. Although rare, some patients
may react to the anesthetic. All patients should be dissuaded from
rubbing their numb eyes, and all patients should be informed of
the devastating consequences of repeated instillation of these anesthetics
for pain. Conjunctival cultures are obtained by lowering the bottom
eyelid and applying the moistened applicator to the lower bulbar
conjunctiva for about 5 seconds without touching the eyelid margin.
The eyelid margins are cultured similarly by applying the moistened
applicator to the eyelashes and margins of both top and bottom eyelids.
It is good practice to culture the conjunctiva and eyelid of both
eyes in cases of conjunctivitis and blepharitis, even if only one
eye is symptomatic.
Conjunctiva
and eyelid specimens can be plated on the same agar medium, but
the laboratory must be able to distinguish between the plants. In
general, squiggles for conjunctiva above the letters R or L for
eyelids are appropriate for distinguishing the location of cultures
on one plate. Transport swabs (culturettes) can also be used but
should be properly marked with sample location. The transport media
should be delivered quickly to the laboratory for media plantation.
Specimen
Collection: Keratitis
Culturing of the cornea is more delicate and should be performed
by an ophthalmologist or experienced physician. A specimen obtained
from a thin cornea by an inexperienced person could lead to a perforated
cornea. Generally the bacteria are located at the leading edge of
an ulcer or infiltrate, and the specimen is obtained with a spatula,
blade, or jeweler's forceps. A corneal specimen could also be obtained
by meticulously dabbing the infected area with a soft-tipped applicator.
Topical anesthetic should always be applied prior to obtaining a
corneal specimen.
Corneal specimens
can be plated on the same agar media with the conjunctiva and eyelid
specimens. A general convention is to form Cs on the media designating
the cornea. Breaking the surface of the agar occurs quite often
and is acceptable. Transport swabs (culturettes) can also be used
but should be properly marked with sample location. The transport
media should be delivered quickly to the laboratory for media plantation.
Specimen
Collection: Endophthalmitis
Intraocular infection is a serious sight threatening disease. Endophthalmitis
generally occurs after surgery (i.e. cataract, keratoplasty) but
can occur after trauma, perforation after severe bacterial keratitis,
and from an endogenous systemic infection. Specimens are obtained
with a syringe and needle by an experienced ophthalmologist who
is aware of all intraocular complications. These specimens are aqueous
and vitreous fluids that are immediately planted separately on 5%
sheep's blood agar, chocolate agar, sabourauds agar with gentamicin,
anaerobic agar medium, and enriched thioglycollate. A few drops of aqueous and vitreous should
be placed on glass slides for gram and giemsa stains. The drops should not be
spread over the slide like a blood smear.
Vitrectomy specimens
are often cultured after endophthalmitis. These specimens are vitreous
diluted with large volumes of BSSplus (50 to 100 ml). Vitrectomy specimens
are concentrated by centrifuging at 3000rpm for 30 minutes. The pellet can be aliquotted onto slides for staining, and to culture media for microbial isolation. Suspended matter in
the diluted vitreous sample could be fished-out, placed on a glass
slide, and stained for the examination of organisms. This is especially
advantageous for a rapid laboratory diagnosis of Propionibacterium
acnes and fungi.

|
 |

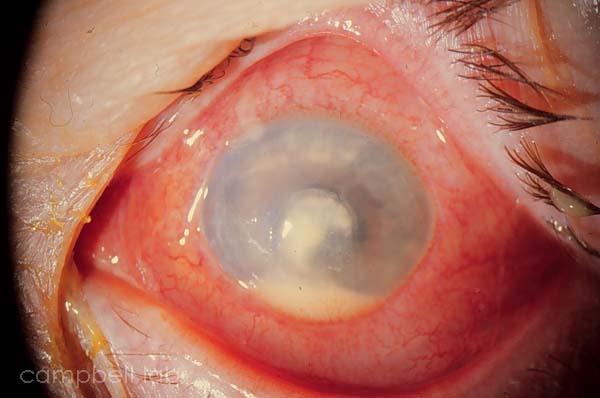
P. aeruginosa corneal ulcer
(Click on image to enlarge)
 |
|

